eCTD Validator - eSubmission Technical Validation
The purpose of validation is to help ensure sponsors provide valid electronic transactions and reduce follow-up times to agency requests. Validation minimizes the possibility of rejection due to technical validation errors, which can cause delays in the overall regulatory process.
Prior to filing your eCTD, (Human) NeeS, Veterinary NeeS or eDok submissions to regulatory agencies you should make certain your sequences are technically valid.
All your usage scenarios are covered:
Use Mono eCTD Validator.
Use Mono eCTD Validator.
Use Mono eCTD Validator.
Use Mono eCTD Validator.
Mono eCTD Validator is a multi-regional eCTD validation solution, packed with powerful features to help you deliver valid electronic submissions to regulatory agencies.
Mono eCTD Validator (Master edition) is a part of Mono eCTD Office.
Mono eCTD Validator is a CESP (Common European Submission Portal) approved validation tool.
Mono eCTD Validator is a suggested validation tool by Australian TGA.
Validation Rules - Complexity Friendlified
Mono eCTD Validator was built with the ease-of-use in mind: the complex list of technical rules is presented in a user-friendly manner. Rules are grouped category; each rule includes a severity level, description, comments and corrective actions explaining how to remedy a detected issue.
Mono eCTD Validator streamlines the technical review process and ensures compliance with eCTD specifications, guidelines, best practices and regulatory requirements across ICH regions.
Mono eCTD Validator's fluent user interface hosts a rule tree and a set of tabbed panes to quickly preview regional metadata (envelopes / administrative info / transaction info), sequence summary and PDF validation reports.
Most recently validated sequences are kept for faster re-validation. Upon validation the validation summary and the complete validation report (along with partial report - including only rules that failed to met the validation criteria) in PDF format is generated.
eCTD Supported Validation Sets
Mono eCTD Validator can technically check any ICH eCTD (including Veterinary NeeS) submission. VNeeS regions supported: CH (eDok), EU, GCC. eCTD regions supported: US, CA, EU, BA, CH, GCC, JO, AU, TH, TN, ZA, UA, SG, WHO, ....
A Validation Set is defined by the Submission Type, Region, Module 1 version and the technical Validation document version.
Mono eCTD Validator automatically detects and selects the correct validation set for the selected sequence to be validated.
In each edition all supported validation sets are available:
Active license holders will receive all application updates, for free, as soon as they are released.
Special: eCTD Product Validation
How Healthy is Your eCTD Product?
Have a "mature" eCTD product? Lots of sequences? Are you sure the entire lifecycle is valid?
A special feature of Mono eCTD Validator: eCTD Product Validation processes *all sequences* for a specified eCTD product and performs most important checks related to the validity of Content (e.g. documents exist?, leaf operation target documents exists?, ...), Lifecycle (e.g. replace operation on deleted leaf?, duplicate modified-file attributes?, ...) and Checksum matching (e.g. have your documents been modified?, have backbone files been modified?, ...).
Both eCTD v 3.2.2. and eCTD v 4.0 supported!Get Your Mono eCTD Validator License
Mono eCTD Validator is available in four editions: Beginner, Professional, Expert and Master. The Beginner edition is FREE and has most limitations and none of extra tools. The Professional edition has most features restricted. The Expert edition has some advanced features restricted. The Master edition has no limitations and includes ALL the in-depth features and eCTD validation tools you might need.
Make sure to get your license before promo expires. All new future application releases will be provided for free to active license holders.
Ready to try the fully functional trial? Request your
free Master edition copy.
Ready to unleash all your potentials? Purchase your license:
If you have an active license of Mono eCTD Office or Mono eCTD Viewer - you can opt for even more discount!
Contact Us.
Mono eCTD Validator Editions
Each edition supports validation of all regions and any combination of submission-type, procedure-type or similar, not tied to a specific country. Never mind if you need to validate an EU centralised procedure or a CDER/FDA IND sequence or a Swissmedic Drug Master File: all validation sets are available in all editions.
Mono eCTD Validator Features
Mono eCTD Validator is under constant testing and development. Features you will benefit from:
Your eSubmission Validation Solution
Even if you already have an eCTD validation tool: try Mono eCTD Validator to unleash all the potentials of eCTD compliance testing.
Make sure your submissions are not rejected by Regulatory Agencies!
Mono eCTD Validator is a CESP (Common European Submission Portal) approved validation tool.
Mono eCTD Validator is a suggested validation tool by Australian TGA.
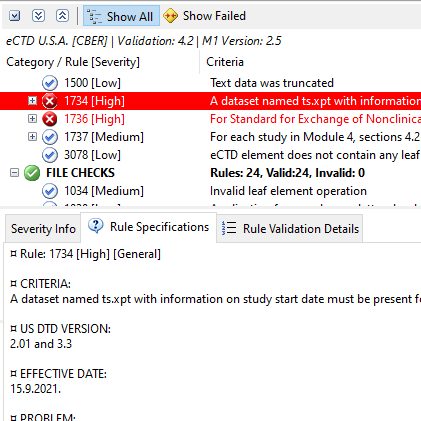
Validation Rules Tree
The appropriate validation set is automatically detected and selected by Mono eCTD Validator. Each validation set defines a group of rules as specified by each regional Agency. Rules are defined by their severity (medium, high ... or pass/fail) and their descriptive text and comments and possible how-to-solve actions. The rule validation details tab lists additional entries related to documents that failed to meet the required criteria.
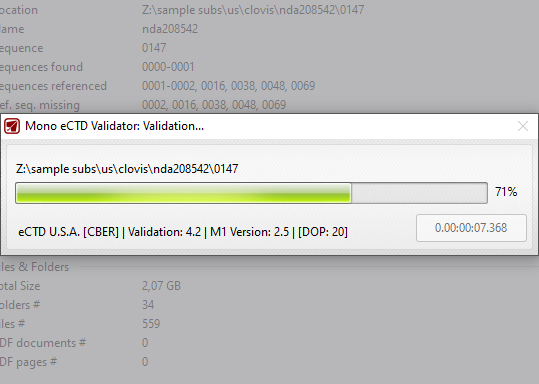
No time to waste time?
Nobody has seconds (or minutes) to spare. Mono eCTD Validator will unleash your machine processing power. During validation, multiple documents will be processed (validated) at the same time. Have your sequence validated in no time. A 4 GB submission validated in less than 4 seconds!
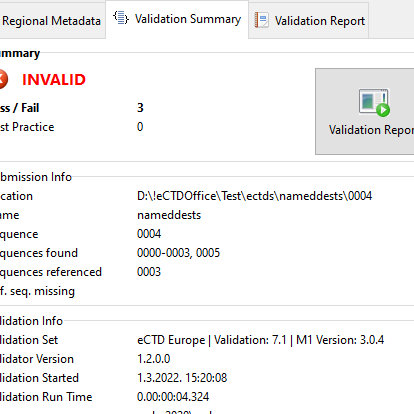
Validation Summary
Your validated sequence at a glance. The Validation Summary provides your starting point to determining if a sequence is valid or not. What's more it lists supplementary data like additional sequences found, sequences referenced, missing sequences. Further: number of files, file types, validation version, ...
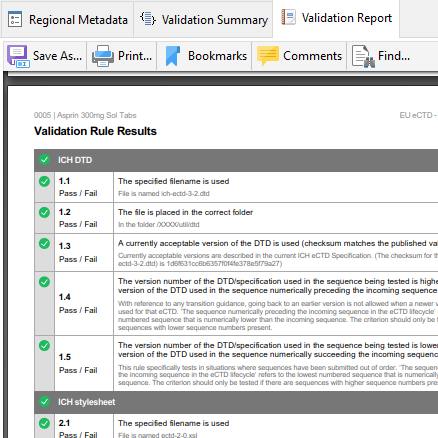
Validation Report: Complete
Upon validation, the complete validation report in PDF format, including results for all validation set rules, is generated. Readily save or print the validation report and send it to your Regulatory Agency or client.
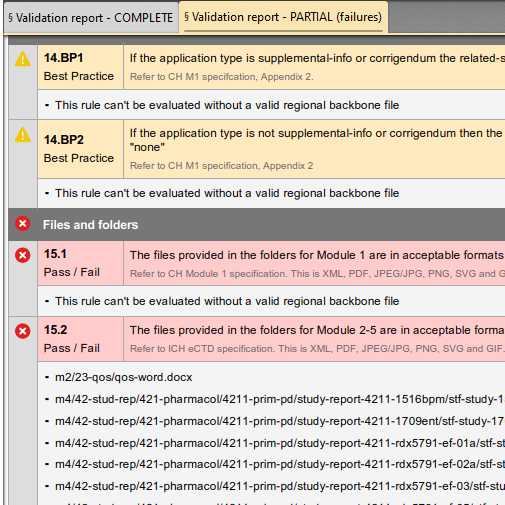
Validation Report: Partial
If there are rules that failed to meet validation criteria, a partial report will also be generated including only the rules which did not succeed. Quickly scan and easily fix your submission.
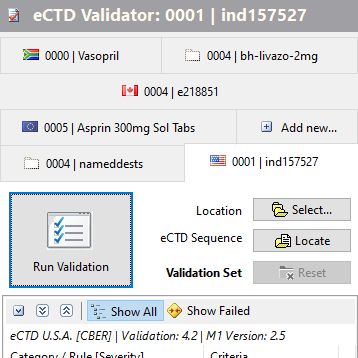
Most Recently Validated
Keep the tabbed list of most recently validated sequences for faster re-validation. Decide what number of items to keep. Each tab hosts the regional image, product name and the sequence number - so you can easily locate the submission you need to re-validate.
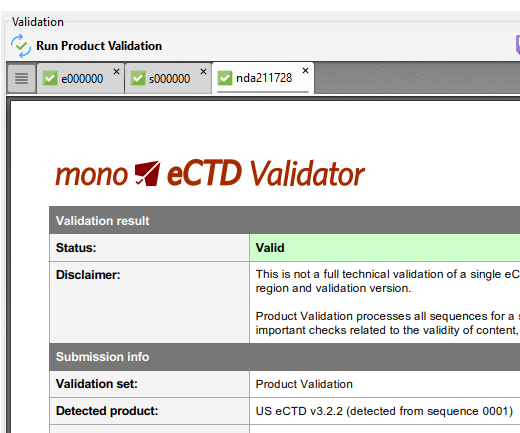
eCTD Product (entire lifecycle) Validation
Not a technical validation of a single eCTD sequence according to the specified region and validation version, BUT: Product Validation processes all sequences for a specified eCTD product and performs most important checks related to the validity of content, lifecycle and checksum matching.
Markup Annotations (/Comments)
Add markup style annotations to the generated validation report. Speed up your internal reviewing process using standard PDF commenting tools: highlight and sticky note. Add replies, set statuses and open in any PDF viewer.
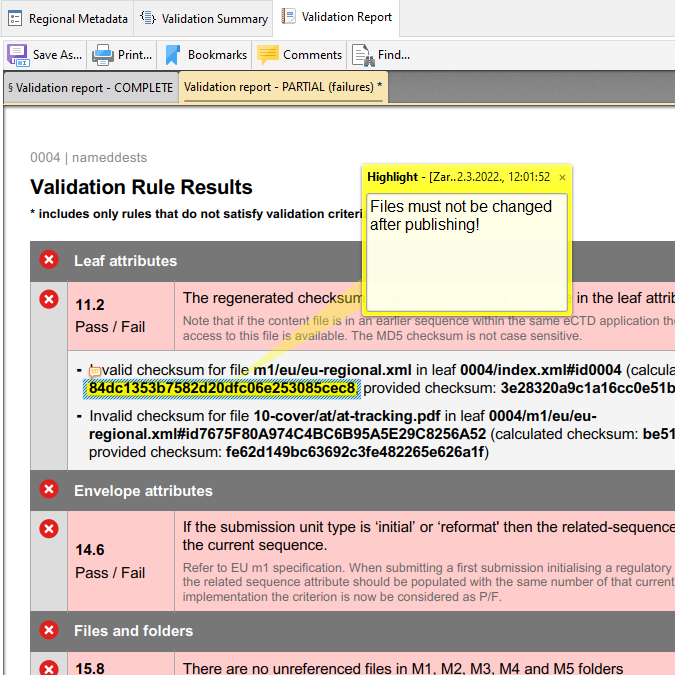
PDF Hyperlink Explorer
PDF Hyperlink Explorer quickly scans and processes PDF documents and hyperlinks to present a clean and simple view of how your documents are cross-linked. Check for the type of the hyperlink (including link text, page, position), target documents, whether a document is optimized for Fast Web View, if PDF properties are valid (initial view magnification, initial view page layout, PDF version, etc.). Process any PDF collection - not only an eCTD/NeeS submission!
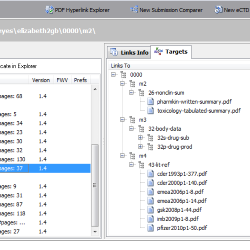
Submission Comparer
Use Submission Comparer to determine changes between two eCTD (/NeeS) sequences, pinpoint missing files, alternations in file size or differences in MD5 checksum between same named documents. Filter the compared display to show only the missing documents in the matching structure.
Contact Us.


